油酸、棕榈酸添加剂诱导HepG2脂肪肝细胞模型
非酒精性脂肪性肝病(non-alcoholic fatty liver disease,NAFLD),又被称为肝脏甘油三酯(TG)过度沉积(>5%肝重),是指除外酒精和其他明确的肝损害因素所致的,以肝脏脂肪变性为主要特征的疾病,包括单纯性脂肪肝、非酒精性脂肪性肝炎、脂肪性肝纤维化、肝硬化以及肝细胞癌。近年来,NAFLD已成为全球最常见的慢性肝病病因之一,每年造成116万人死亡。“双重打击”理论认为,NAFLD的发病机制是由肝细胞中脂质堆积+氧化应激所致。
一篇发表在Toxicology 2021上的文章(The combined impact of decabromodiphenyl ether and high fat exposure on non-alcoholic fatty liver disease in vivo and in vitro),使用了油酸钠+棕榈酸钠组合诱导HepG2脂肪肝细胞模型,具体如下:
作者采用100-1600 μM范围内不同浓度的棕榈酸钠(Sodium palmitate)处理HepG2细胞,维持24或48小时,可见棕榈酸钠浓度依赖性地降低HepG2细胞活力(图1B),据此计算出棕榈酸钠的半抑制浓度IC50=490μM。然后作者采用0.25×IC50、0.50×IC50或0.75×IC50的棕榈酸钠处理HepG2细胞,发现0.25×IC50棕榈酸钠对HepG2细胞活力无显著影响(图1C),从而挑选出0.25×IC50(即122.5μM)棕榈酸钠,联合2倍浓度(245μM)的油酸钠,用于下一步研究。
作者进一步采用油酸钠+棕榈酸钠(245μM+122.5μM,简称HF)处理HepG2细胞,结果显示该组合可以增加HepG2细胞中脂滴形成和甘油三酯(TG)含量(图2),增加活性氧簇(ROS)和丙二醛(MDA)含量(图3),并减少线粒体数量,增加线粒体损伤(图4)。证明低浓度油酸钠+棕榈酸钠2:1组合处理2天可以成功构建脂肪肝细胞模型,并导致肝细胞氧化应激和线粒体损伤。
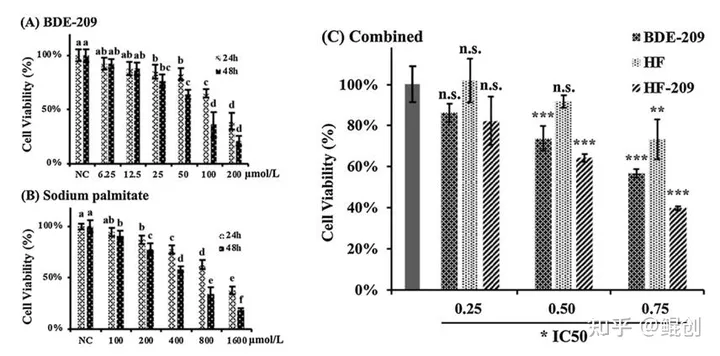
图1. 棕榈酸钠、油酸钠+棕榈酸钠(HF)降低HepG2细胞活力。A. 不同浓度BDE-209对HepG2细胞活力的影响;B. 不同浓度棕榈酸钠对HepG2细胞活力的影响;C. BDE-209、油酸钠+棕榈酸钠(HF)、联合HF和BDE-209(HF-209)对HepG2细胞活力的影响。
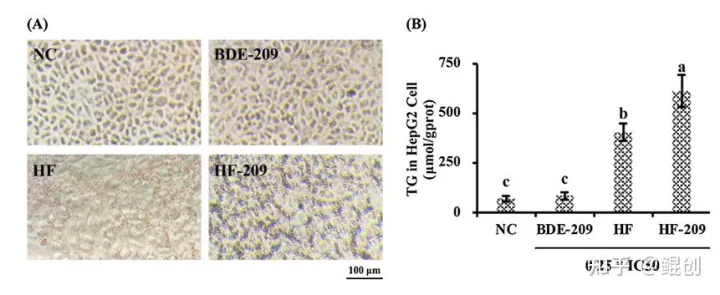
图2. 油酸钠+棕榈酸钠(HF)诱导HepG2脂质堆积。A. HepG2细胞经HF(油酸钠+棕榈酸钠=245+122.5μM)处理48小时后的油红O染色照片。B. HepG2细胞内甘油三酯(TG)含量测定。
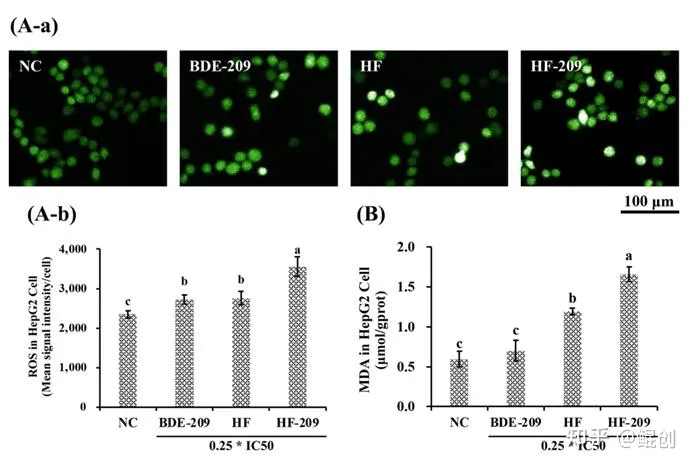
图3. 油酸钠+棕榈酸钠(HF)诱导HepG2氧化应激。A. HepG2细胞经HF(油酸钠+棕榈酸钠=245+122.5μM)处理48小时后的DCFH-DA染色照片(A-a)和活性氧簇水平(ROS,A-b)。B. HepG2细胞内丙二醛(MDA)含量测定。
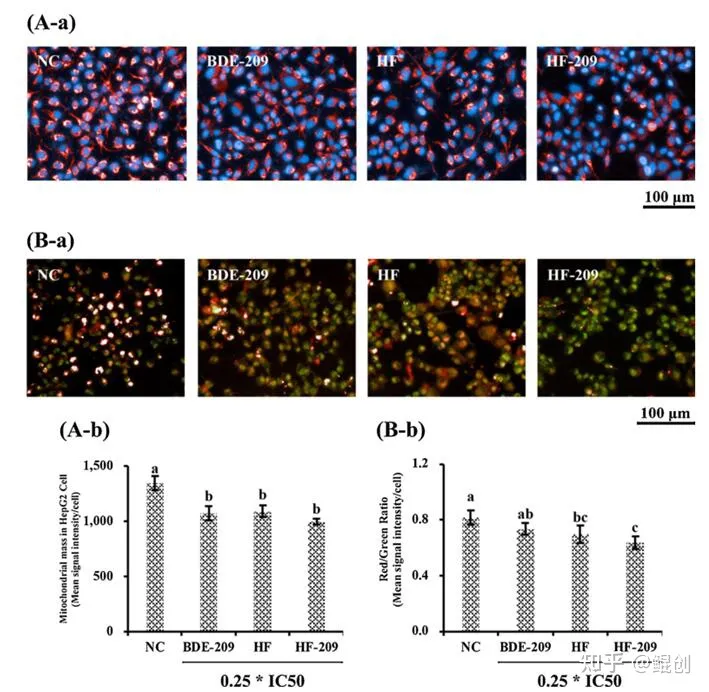
图4. 油酸钠+棕榈酸钠(HF)诱导HepG2线粒体损伤。A. HepG2细胞经HF(油酸钠+棕榈酸钠=245+122.5μM)处理48小时后的MitoTracker染色照片(A-a)和线粒体数量(A-b)。B. JC-1染色照片(B-a)和红色/绿色荧光比值(B-b)。
小编总结:油酸高脂试剂盒(油酸钠+棕榈酸钠)主要用于诱导脂肪肝细胞模型,一般采用的浓度比为油酸钠:棕榈酸钠=2:1,处理时间2-3天。本研究采用245μM油酸钠和122.5μM棕榈酸钠处理2天,在未导致HepG2细胞活力明显下降的情况下,成功诱导了脂肪肝细胞模型,具有较好的参考价值。
其他参考及相关文献:
1)Cardiac-specific CGI-58 deficiency activates the ER stress pathway to promote heart failure in mice
Palmitic acid (PA) and its solvent (vehicle) were purchased from Kunchuang Biotechnology (Xi’an, Shanxi, China), in which PA was coupled to FA-free bovine serum albumin (BSA) in a ratio of 2mM PA:3% BSA
2)High-fat diet impairs ferroptosis and promotes cancer invasiveness via down regulating tumor suppressor ACSL4 in lung adenocarcinoma
Acid palmitate was obtained from Xian Kunchuang Science and Technology Develop Co. Ltd (Xian, China)
3)Lipid-induced DRAM recruits STOM to lysosomes and induces LMP to promote exosome release from hepatocytes in NAFLD
Oil red O staining and intracellular triglyceride measurement For FA treatment, 0.3 mM PA or bovine serum albumin solution (Kunchuang Biotechnology, Xian, China) was incubated with the cultured HepG2 cells.
4)Monocyte-derived extracellular vesicles upon treated by palmitate promote endothelial migration and monocytes attachment to endothelial cells
Saturated FFA palmitate (16: 0) and its solvent(Cat. No. SYSJ001) were purchased from Kunchuang biotechnology.
5)Optimization of Porphyran Extraction from Pyropia yezoensis by Response Surface Methodology and Its Lipid-Lowering Effects
Palmitic acid (PA) was provided by Kunchuang Biotechnology (Xi’an, China). All other chemical reagents used were of analytical grade.
6)The combined impact of decabromodiphenyl ether and high fat exposure on non-alcoholic fatty liver disease in vivo and in vitro
Sodium palmitate and sodium oleate were purchased from Kunchuang Technology Development Co., Ltd. (Xian, China).
7)The impact of sitagliptin on macrophage polarity and angiogenesis in the osteointegration of titanium implants in type 2 diabetes
Endothelial cell medium containing 25 mmol/L glucose and 500 mmol/L bovine serum albumin-conjugated palmitate (Kunchuang Biotechnology, Xi’an, China, Cat#: SYSJ001) was used as the mimic milieu of type 2 diabetes (high glucose and fat) which accounts for over 90 % cases in diabetic patients .
8)Wanhao Gao 1, Xingchen Guo 1,et al. Monocyte-derived extracellular vesicles upon treated by palmitate promote endothelial migration and monocytes attachment to endothelial cells.
Biochemical and Biophysical Research Communications .2020 May:685-691.
9)Ji L, Liu F, Jing Z, et al. MICU1 Alleviates Diabetic Cardiomyopathy Through Mitochondrial Ca2+-Dependent Antioxidant Response. Diabetes. 2017 Jun;66(6):1586-1600
10)Yan W, Zhang H, Liu P, et al. Impaired mitochondrial biogenesis due to dysfunctional adiponectin-AMPK-PGC-1α signaling contributing to increased vulnerability in diabetic heart. Basic Res Cardiol. 2013 May;108(3):329.



